About HHTU
The HHTU along with the Methods Hub is a part of the infrastructure in the Institute for Clinical and Applied Health Research (ICAHR). ICAHR is a world-leading research hub which brings together expertise from the Faculty of Health Sciences at the University of Hull and Hull York Medical School to support research across a range of specialist areas.
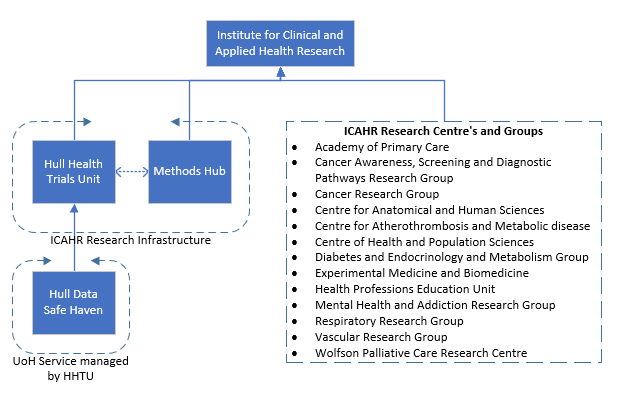
The Hull Health Trials Unit provides a range of practical and specialist support throughout the clinical research process, helping researchers at all stages of their study;
- Study Design and Funding Applications
- Study Management including Regulatory Approvals
- Data Management with secure online eCRF and Randomisation
- Data Safe Haven for storage and processing of Sensitive Health Data
- Statistical Analysis and Reporting

We work with you from the early stages of your ideas, providing input to; trial design, feasibility, sample size and statistical analysis, qualitative (mixed methods) components, ethics and regulatory requirements, costings for proposals, resource planning and timelines, funding applications.
We help researchers to build their capacity by leading the set-up and management of trials. Experienced trial management staff will input to; protocol development, PPI participation, risk assessments, obtaining regulatory approvals, processing amendments, site contracting, site set-up, trial QA and site monitoring, trial safety reporting and pharmacovigilance, coordinating trial logistics, budget management, study reporting and coordinating trial oversight committee meetings.


Working with our data management team, you can access a suite of regulatory compliant information systems to allow the efficient development and delivery of your research project. We will work with you to; design workflows, eCRF and database, coordinate data queries and data cleaning, export data for reporting and analysis.
We use a regulatory compliant cloud based electronic data capture system, REDCap Cloud. Data can be entered remotely by your study staff or participants from any computer or device (tablets and mobile), facilitating efficient and accurate data collection. The system can support blinded randomisation from simple blocks through to complex minimisation. REDCap incorporates; eCRF, patient information, eConsent, randomisation, surveys, data import and reporting for a seamless path from first contact through to follow up in one system.
Our DSH is a safe and secure environment for storage and processing of data sets requiring compliance with NHS Information Governance (DSP) toolkit standards. We provide a controlled environment, access to all necessary software and the option of support from HHTU data specialists to help with linkage and anonymisation.


We provide expert statistical collaboration for all HHTU trials and can develop your analysis plan. Our statistical input into your research is vital to ensure that your data is collected and analysed accurately and most importantly will ensure your results are publishable in high impact journals.
Throughout your trial we can assist with; preparation and submission of progress reports, amendments and close out reports to funding body/sponsor; MHRA; research ethics committees; R&D departments in participating NHS trusts. We can host trial webpages, create newsletters and contribute to, and coordinate the publication of results.

