HHTU News Roundup
CONIFAS2 is live
We’re delighted to share that Co-produced nature-based intervention feasibility for children with ADHD Study (CONIFAS2) is now open to recruitment! This study is led by Dr Hannah Armitt with Kathryn Date managing the trial at HHTU.
CONIFAS2 is intervention is a home-based, family-led intervention delivered with professional third-party telephone support. It encourages daily engagement with nature for at least 10 minutes, including activity cards and supportive psychoeducational elements. CONIFAS2 is ready to be tested in NHS funded neurodiversity services assessing the feasibility and acceptability of a full-scale effectiveness trial.
Protocol Paper Published
DOORStep have a newly published protocol paper in BMJ Open. This study assesses whether offering free, bookable, door-to-door transport to and from breast cancer screening appointments could increase screening attendance. This cluster-randomised GP feasibility trial is funded by Yorkshire Cancer Research, led by Chief Investigator Charlotte Kelly, and managed by Mahboobeh Haji Sadeghi at the Hull Health Trials Unit. The photo below is DOORStep team member outside a breast screening van where attendees were encouraged to complete a travel survey. This stage of the study is complete and analysis is now taking place. We look forward to reading the results paper when it’s ready!

MABEL Video live
Following on from our MABEL Study Spotlight, a new patient information video has now been released. It was co-created with our patient and public involvement group at the University of Hull for people who have been recommended to try low-dose morphine for their breathlessness by their doctor. Using clear animation and narration, the video covers living with breathlessness, why morphine might help, how it is taken, and addresses common concerns in an accessible and reassuring way. The messages in the video are consistent with the evidence from the MABEL study.
Chronic breathlessness is often disabling despite best treatments of the underlying condition(s) that cause the breathlessness. MABEL tested if regular, low dose morphine capsules regularly twice a day are better than placebo (dummy) ones for chronic breathlessness and whether morphine improves daily activity. It was found that people taking morphine on average completed about 10 more minutes of moderate-vigorous exercise per day. This was a study lead collaboratively by teams at the University of Hull and the University of Edinburgh and was funded by NIHR HTA.
You can watch the video and also read more about the MABEL study here and the video was created by graphic designer Amymadethis.
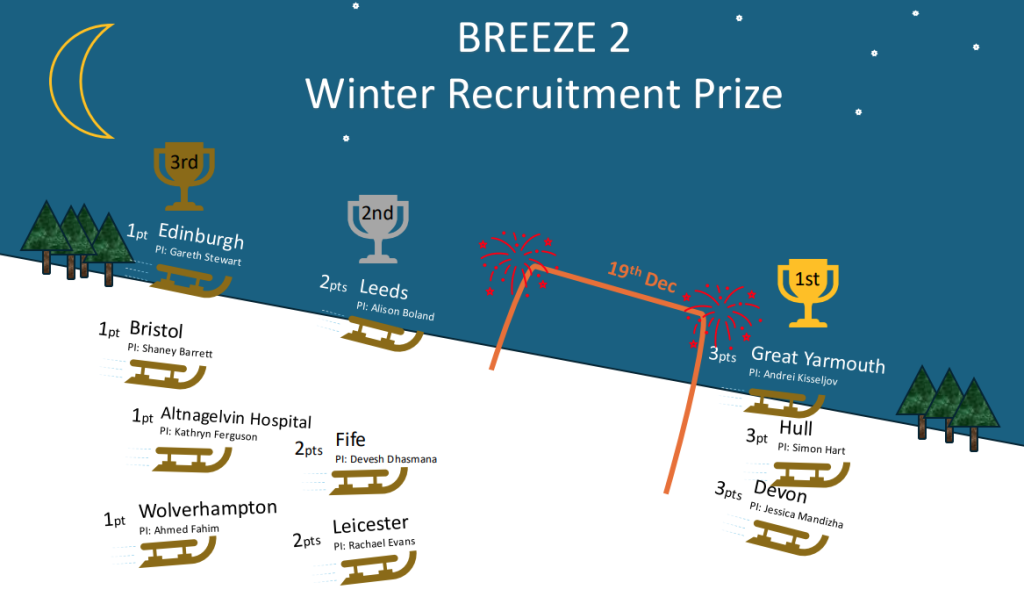
Other January project headlines:

2 participants recruited

4 participants consented

6 participants recruited

3 participants recruited

8 participants recruited

STARLIT 3 has recruited 2 participants

2 sites opened